Codeine/paracetamol
This article needs additional citations for verification. (July 2012) |
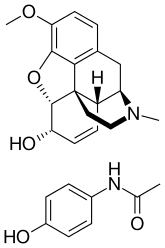 | |
| Combination of | |
|---|---|
| Codeine | Opioid analgesic |
| paracetamol | Anilide analgesic |
| Clinical data | |
| Trade names | Tylenol with codeine, others |
| MedlinePlus | a601005 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| | |
Codeine/paracetamol, also called codeine/acetaminophen and co-codamol, is a compound analgesic, comprising codeine phosphate and paracetamol (acetaminophen). Codeine/paracetamol is used for the relief of mild to moderate pain when paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs; such as ibuprofen, aspirin, and naproxen) alone do not sufficiently relieve symptoms.[2][3]
In 2022, it was the 166th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[7][8]
Side effects
[edit]The most common side effects include constipation, nausea and drowsiness.[9] Others include coughing up blood from the lungs, skin rashes, dizziness, sedation, shortness of breath, hypersensitivity reaction, fainting (syncope or near syncope), confusion, loss of short-term memory, changes in blood, allergic reactions, euphoria, dysphoria, abdominal pain, itchiness, easy bruising, bleeding gums, vivid dreams, dry mouth and addiction.[10]
Genetic differences between people cause differing rates of metabolism of codeine to morphine. In about 5% of people this may happen particularly fast, causing morphine to be passed through breast milk in amounts that may cause fatal respiratory depression in a breastfed baby.[11]
Society and culture
[edit]Availability
[edit]Of the European Union (EU) member states, 11 allow over-the-counter sale of solid dosage forms of codeine, including codeine/paracetamol; they are Bulgaria, Cyprus, Denmark, France, Ireland, Latvia, Lithuania, Malta, Poland, Romania and Slovenia.[12]
References
[edit]- ^ "Acetaminophen with Codeine Product information". Health Canada. 25 April 2012. Archived from the original on 13 September 2022. Retrieved 13 September 2022.
- ^ a b "Boots Paracetamol & Codeine 500mg/8mg Tablets - Patient Information Leaflet (PIL)". (emc). 30 November 2021. Archived from the original on 18 July 2022. Retrieved 17 July 2022.
- ^ a b "Co-Codamol 15/500 Tablets - Summary of Product Characteristics (SmPC)". (emc). 25 March 2021. Archived from the original on 5 August 2021. Retrieved 17 July 2022.
- ^ "Controlled Substances - Alphabetical Order" (PDF). Archived (PDF) from the original on 21 April 2021. Retrieved 29 June 2024.
- ^ "Acetaminophen and Codeine Phosphate tablet". DailyMed. 31 July 2020. Archived from the original on 18 March 2022. Retrieved 17 July 2022.
- ^ "Acetaminophen and Codeine Phosphate solution". DailyMed. 25 May 2022. Archived from the original on 11 May 2021. Retrieved 17 July 2022.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Acetaminophen; Codeine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Co-codamol for adults: painkiller containing paracetamol and codeine". National Health Service. 19 December 2018. Archived from the original on 14 January 2022. Retrieved 16 April 2021.
- ^ Nielsen S, Van Hout MC (2017). "Over-the-Counter Codeine-from Therapeutic Use to Dependence, and the Grey Areas in Between". Current Topics in Behavioral Neurosciences. 34: 59–75. doi:10.1007/7854_2015_422. hdl:1959.4/unsworks_62273. ISBN 978-3-319-60014-7. PMID 26768736.
- ^ "Codeine Use While Breastfeeding May Be Dangerous". CTV News. 20 August 2008. Archived from the original on 1 September 2008. Retrieved 13 August 2020.
- ^ Foley M, Harris R, Rich E, Rapca A, Bergin M, Norman I, et al. (November 2015). "The availability of over-the-counter codeine medicines across the European Union". Public Health. 129 (11): 1465–1470. doi:10.1016/j.puhe.2015.06.014. PMID 26215740. Archived from the original on 25 March 2022. Retrieved 8 July 2020.
